Lecture
Ionistor (supercapacitor, ultracapacitor, double-layer electrochemical capacitor, English EDLC, Electric double-layer capacitor ) is an electrochemical device, a capacitor with an organic or inorganic electrolyte, which has a double electrical layer at the interface between the electrode and the electrolyte. Functionally, it is a hybrid of a capacitor and a chemical current source.
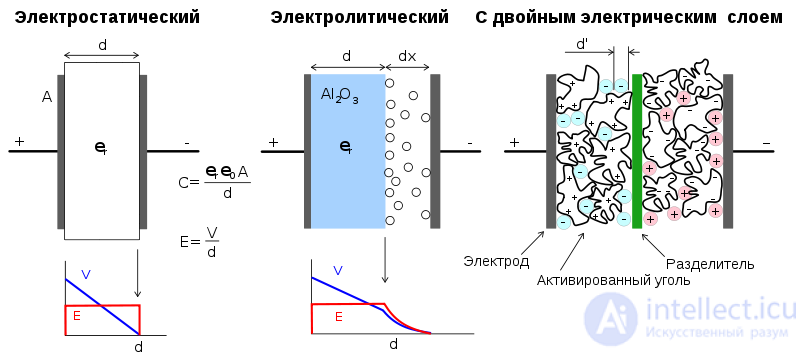
Comparison of the design schemes of the three capacitors. Left: "normal" capacitor, in the middle: electrolytic, right: ionistor
Due to the fact that the thickness of the electrical double layer (that is, the distance between the “plates” of the capacitor) is extremely small, the energy stored by the ionistor is higher compared to conventional capacitors of the same size. In addition, the use of the electric double layer instead of the usual dielectric allows to significantly increase the surface area of the electrode. A typical capacity of an ionistor is several pairs, with a nominal voltage of 2-10 volts.
The first double layer capacitor on porous carbon electrodes was patented in 1957 by General Electric [1] . Since the exact mechanism at that point in time was not clear, it was assumed that energy is stored in the pores on the electrodes, which leads to the formation of an “exceptionally high charge storage capacity” . A little later, in 1966, Standard Oil of Ohio, Cleveland (SOHIO), USA patented an element that stored energy in a double layer. [2]
Faced with the fact of a small sales volume, in 1971 SOHIO transferred the license to NEC, which managed to successfully promote the product on the market under the name “Supercapacitor” (Supercapacitor). In 1978, the Panasonic company launched the “Gold capacitor” (“Gold Cap”) “Golden Capacitor” operating on the same principle. These capacitors had a relatively high internal resistance, limiting energy returns, so these capacitors were used only as storage batteries for SRAM.
The first ionistors with low internal resistance for use in high-power circuits were developed by PRI in 1982. These ionistors appeared on the market under the name "PRI Ultracapacitor".
1) Ionistors with perfectly polarized carbon electrodes (“ideal” ionistor, ionic capacitor). Do not use electrochemical reactions, working by ion transfer between the electrodes. Some variants of the electrolyte: 30% aqueous KOH solution; 38% aqueous solution of H 2 SO 4 ; organic electrolytes. [3]
2) Ionistors with a perfectly polarized carbon electrode and non-polarizable or weakly polarized cathode or anode (“hybrid” ionistors). An electrochemical reaction takes place at one electrode. Options: Ag (-) and solid electrolyte RbAg 4 I 5 ; 30% aqueous solution of KOH and NiOOH (+) [3]
3) Pseudocapacitors - ionistors using reversible electrochemical processes on the surface of electrodes. Have a high specific capacity. Electrochemical scheme: (-) Ni (H) / 30% aqueous solution of KOH / NiOH (+); (-) C (H) / 38% aqueous solution of H 2 SO 4 / PbSO 4 (PbO 2 ) (+). [3]
With the advent of ionistors, it became possible to use capacitors in electrical circuits not only as a transforming element, but also as a voltage source. Widely used as a replacement battery for storing information about the parameters of the product in the absence of external power. Such cells have both several advantages and disadvantages over conventional chemical current sources - galvanic cells and batteries:
Electrodes are usually performed by using porous materials, such as activated carbon or foamed metals, instead of conventional insulation materials. The total surface area, even in the thin layer of such a material, is many times larger than in traditional materials, such as aluminum, which made it possible to store charge in any volume.
The energy density of ionics is still several times less than the battery capacity. For example, the energy density of the ionistor BCAP3000 3000F x 2.7V weighing 0.51 kg is 21.4 kJ / kg. This is 7.6 times less than the energy density of lead electrolytic batteries, 25 times less than lithium-polymer batteries, but ten times more than the energy density of an electrolytic capacitor.
The power density of the ionistor depends on the internal resistance. In the latest models of ionistors, the internal resistance is quite small, which allows to obtain power comparable to the battery.
In 1997, researchers at CSIRO developed a super-capacitor that could store a large charge through the use of film polymers as a dielectric. Electrodes were made of carbon nanotubes. For conventional capacitors, the specific energy is 0.5 W · h / kg, and for PET capacitors, it was 4 times higher. [ source not specified 1693 days ]
In 2008, Indian researchers developed a prototype of an ionistor based on graphene electrodes with a specific energy capacity of up to 32 Wh / kg, comparable to that for lead-acid batteries (30-40 Wh / kg) [5] .
In 2011, Korean scientists under the guidance of Professor Choi Jung Wook (Choi Jung-wook) developed a supercapacitor made using graphene and nitrogen, which provides double the capacity compared to traditional energy sources of the same class. Improving the electrical properties of the battery was achieved by adding nitrogen. [6]
Heavy and public transport
Currently, buses with ionistor power are manufactured by Hyundai Motor and Trolza.
Buses on ionisators from Hyundai Motor are ordinary buses with electric drive, powered by onboard ionistor. As conceived by designers from Hyundai Motor, such a bus will be charged at every second or every third stop, and the stopping time is enough to recharge bus ionistors. Hyundai Motor positions its bus on ionistors as an economical alternative to a trolley bus (there is no need to lay a contact network) or a diesel (and even hydrogen) bus (electric power is cheaper than diesel or hydrogen fuel).
Buses on ionistors from "Trolza" technically are "rodless trolley buses." That is, it is structurally a trolley bus, but without power supply rods from a contact network and, accordingly, with power supply to the electric drive from ionistors.
But ionistors are particularly promising as a means of implementing an autonomous running system for ordinary trolleybuses. A trolley-bus equipped with ionistors approaches maneuverability from the bus. In particular, such a trolley bus can:
Thus, the trolleybus system, operating the trolleybuses equipped with ionistors, in terms of flexibility approaches that of a regular bus.
Automotive
E-Mobile - a car project developed in the Russian Federation, used a supercapacitor as the primary means for the accumulation of electrical energy. These supercapacitors themselves are not yet commercially available and have been developed in parallel with the car.
There are projects that combine a supercapacitor and a chemical accumulator in a single unit, which mutually compensates for the shortcomings of both. The result is a drive with a long service life, lower cost and more energy than with conventional batteries.
Auto racing
The KERS system used in Formula 1 uses precisely ionistors.
Used for main and backup power in flashlights, flashlights, handheld players and automatic utility meters - wherever you need to quickly charge the device. The laser detector of breast cancer on ionistors charges in 2.5 minutes and works for 1 minute. [7]
In 2007 they released a screwdriver in which ionistors with a total capacity of 55 farads are charged for 1.5 minutes. The charge is enough for 22 screws.
In auto accessories stores, ionistors with a capacity of about 1F are sold to power the car stereo (and equipment powered from the cigarette lighter socket) with the ignition off and during engine start (on many vehicles, all other consumers are disconnected while the starter is working), and also to smooth out voltage surges with peak loads, for example, powerful speakers.
According to the 2006 MIT staff [8] , ionistors may soon replace conventional batteries. In addition, in 2009, an ionistor based battery was tested in which iron nanoparticles were introduced into the porous material. The resulting electrical double layer of transmission electrons is twice as fast due to the creation of a tunnel effect. A group of scientists from the University of Texas at Austin has developed a new material, which is a porous bulk carbon. The carbon thus obtained had the properties of a supercapacitor. Processing the above material with potassium hydroxide led to the creation in carbon of a large number of tiny pores, which, in combination with the electrolyte, were able to store a huge electric charge. [9]
In 2013 it became known about the development at the facilities of the Novosibirsk Plant of Radio Components Oxide, a member of the Ruselectronika holding, of the first Russian ionistors [ source not specified 37 days ] . The company's goal is to implement a venture project for the purchase of a production line and the organization of mass production ionistors, which would require an investment of $ 6,624,000. [10]
In June 2014, the scientists of the Institute of Solid State Chemistry and Mechanochemistry of the SB RAS and the company Yarovit Engineering joined the development of the Russian ionistor.
At present, one of the necessary parts of the capacitor has been created - a solid nanocomposite electrolyte with conductivity for lithium ions. The development of electrodes for the capacitor. One of the tasks is to reduce the size of the ionistor due to the internal structure. [eleven]
Comments
To leave a comment
Electronics, Microelectronics, Element Base
Terms: Electronics, Microelectronics, Element Base