Lecture
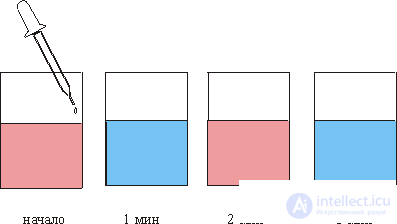
Far from equilibrium, dissipative spatial and temporal structures arise, that is, a nonequilibrium order is formed. Sometimes this order consists in the appearance of oscillations and waves, for example, in chemical dissipative systems. This effect is especially vivid in the so-called Belousov-Zhabotinsky (BZh) reaction: a pink liquid is poured into a glass, into which droplets of colorless liquid are fed from a pipette and the solution in the glass turns blue after another minute, after another minute the liquid itself becomes pink again and t dd (fig. 1). There appear to be a chemical clock.
This phenomenon was discovered in 1951 by experimental chemist B. P. Belousov, a retired commander, head. laboratory at the Institute of Biophysics. Later, in 1959, A. M. Zhabotinsky studied this reaction in detail and gave a qualitative explanation of it. Mathematical modeling of similar processes in 1970 held in England Turing. For a set of studies of reactions of this type, B. P. Belousov and A. M. Zhabotinsky in 1980 were awarded the Lenin Prize. This is a brief history associated with the world famous reaction of the BZ, and now turn to the chemical mechanism of this phenomenon.
In a simplified scheme, the Belousov reaction consists of two stages. In the first stage, the trivalent cerium Ce 3+ (pink color) is oxidized with bromic acid HBrO 3 , which leads to an excess of Ce 4+ ions (blue color):
 Ce 4+ .
Ce 4+ . In the second stage, tetravalent cerium Ce 4+ is reduced by an organic compound, malonic acid (MK), i.e.
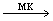 CE 3+
CE 3+ and blue is replaced by pink. This process continues: pink, blue, pink, blue, etc., with a frequency of 1 min (Fig. 2). The periodic process is terminated after a large number of periods due to the irreversible consumption of BrO 3 bromate.
Zhabotinsky described a wide class of chemical wave phenomena in which spatio-temporal order was observed. In this case, both one-dimensional reactions in thin tubes and two-dimensional processes (thin layers of solution between the plates) were realized.
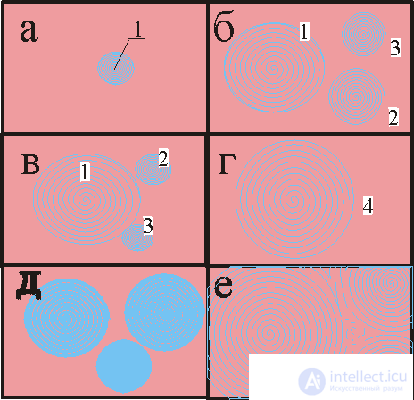 Fig. 3. Sequential frames of wave chemical processes in thin layers of a solution (two-dimensional systems).
Fig. 3. Sequential frames of wave chemical processes in thin layers of a solution (two-dimensional systems).
In fig. 3 shows the development of a wave during the flat realization of the phenomenon: first (a) a color change center 1 appears, it appears due to a local concentration fluctuation; at the same time (b) new concentration centers 2 and 3 appear, the latter can then be absorbed (c) by waves from center 1 and contribute to the development of (d) wave concentration structure 4. Option (d and e) for a more complex picture from many initial ones is possible centers.
Spatio-temporal ordering can be considered as self-oscillatory and autowave processes. These processes are supported by the entropy drain from the system. At the same time, spiral waves can form, they are called reverberators. This type of formation is quite common in biological systems, for example, in the structure of lichens.
The works of I. Prigogine emphasize the connection between the physicochemical processes in open non-equilibrium processes and biological orderliness.
Comments
To leave a comment
Synergetics
Terms: Synergetics