Lecture
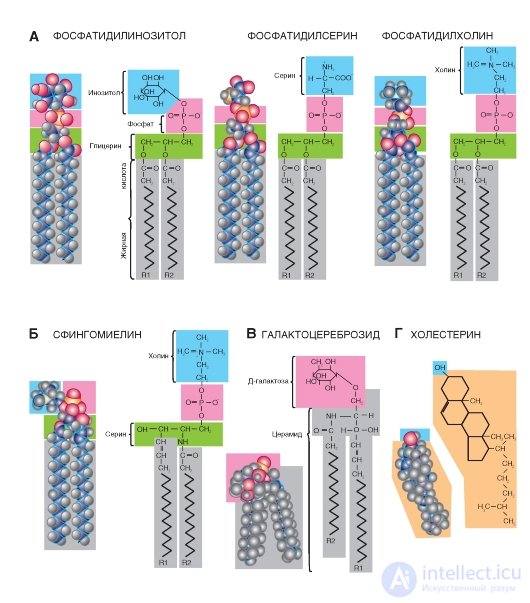
Such phosphoglycerides (Fig. 1-2 A), such as phosphatidylethanolamine, are found in the greatest amount in the body of humans and animals. In addition, phosphatidylserine, phosphatidylinositol, and phosphatidylcholine are present (Figure 1-3 A). They are metabolically related to each other and contain as the X-group aminoalcohols ethanolamine (phosphoethanolamine residue) and choline (phosphocholine residue). In phosphatidylserine, phosphoric acid is esterified with a hydroxyl group of serine phosphoserine. In phosphatidylinosite, the X-group is the six-carbon sugar alcohol inositol (the residue of inositol phosphate ester). In phosphatidylglycerol, the role of the X-group is played by the residue of glycerophosphate.
The sugar molecule can also play the role of the polar X-group of phosphatides. These glycophosphoglycerides, or phosphatidyl sugars, are found in plants and microorganisms. They should not be confused with glycolipids, in the molecule of which sugar is also present, but phosphoric acid is absent.
The structural basis of sphingosine-substituted lipids is the amino alcohol sphingosine. Sphingolipids are found in the membranes of plant and animal cells; especially nervous tissue, in particular the brain. In fatty deposits, sphingolipids are very few. During hydrolysis of sphingolipids, one molecule of fatty acid, one molecule of the unsaturated amino alcohol sphingosine or its saturated dihydrosphingosine analogue, one molecule of phosphoric acid, and one molecule of alcohol (X-OH) are formed. Glycerol sphingolipids do not contain.
The most common sphingolipid sphingomyelin (Fig. 1-3 B), containing choline as the X-group. For sphingomyelin, as well as for all other sphingolipids, it is characteristic that one of its hydrocarbon tails is a long aliphatic chain of sphingosine, and the other is esterified fatty acid. Thus, the conformations of sphingolipids are very similar to phosphoglycerides, since their molecules also contain a polar head and two non-polar tails.
In glycolipids, the head molecules form polar hydrophilic carbohydrate groups, most often D-galactose. However, unlike sphingolipids, glycolipids do not contain
phosphoric acid. The simplest glycolipids include glycosyldiacylglycerols found in plants and microorganisms. Another group, cerebrosides, can be attributed to both glycolipids and sphingolipids, since these compounds contain both sugar and sphingosine. Therefore, they are called cerebrosides, or glycosphingolipids. The content of cerebrosides is especially high in the membranes of nerve cells, in particular in the myelin sheath. Fatty acids found in cerebrosides are unusual because they contain 24 carbon atoms. The most common neuronal, cerebronic and lignoceric acid. The sugar-free sphingosine fatty acid ester is called ceramide.
Steroids are very different in structure from the molecules of other lipid subclasses. Steroids are derivatives of perhydrocyclopentane-phenanthrene cores containing three condensed cyclohexane rings connected to each other. Several hydroxyl polar groups can be attached to these structures, but their number is not enough to make the steroid water soluble.
Important natural steroids include bile acids, male and female sex hormones, adrenal hormones, and some steroids with high biological activity, which include, in particular, some poisons. In cells, these compounds are mostly present in trace amounts, and only representatives of one class of steroids, namely sterols, are an exception. Sterol cells are very rich. These compounds contain an alcoholic hydroxyl group at C-3 and a branched aliphatic chain of eight or more carbon atoms at C-17. They exist either in the form of free alcohols or in the form of ethers, in which the hydroxyl group at C-3 is esterified with a long chain fatty acid.
Examples of steroids: cholesterol (Fig. 1-3 G), cortisol (synthesized in the adrenal glands), female (estrogen) and male (testosterone) sex hormones secreted by gonads. The most common cholesterol, which is contained in the body in both free and esterified form. It is part of the membranes.
Fig. 1-3. The most common membrane lipids.
A - examples of phospholipids; B - an example of sphingolipid; B is an example of cerebroside (glycosphingolipid); G is an example of steroids.
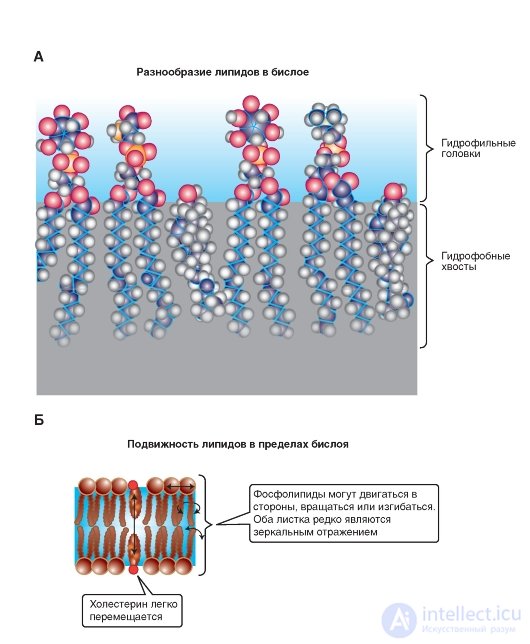
Under physiological conditions, the membranes have dynamic properties. The lipid bi layer (Fig. 1-4 A) is essentially a viscous fluid and is characterized by fluidity. Fluidity is a macroscopic characteristic of the entire lipid bilayer; its value is inverse to viscosity. Since the lipid bilayer is fluid, it therefore has a low viscosity. Depending on the temperature and chemical composition of the membrane, fluidity can be high or low. The fluidity of the lipid bilayer is of little importance in warm-blooded animals, since the temperature of the internal environment of the body is more or less constant, but in cold-blooded animals, this indicator is of fundamental importance, since the viscosity of the lipid bilayer increases with decreasing temperature and, consequently, fluidity decreases. Such changes in the dynamic properties of the membrane, along with a number of others, lead to the fact that at low temperatures the life of cold-blooded animals stops.
However, the viscosity of the phospholipid bilayer is due to two types of mobility at the level of phospholipid molecules, which have two
types of movements: intramolecular and intermolecular (Fig. 1-4 B).
The intramolecular mobility refers to the mobility of the chains of fatty acids and sections of the polar head associated with the flexibility of the chains. Flexibility, in turn, is associated with the possibility of rotation of chemical groups with respect to a single bond. This is the so-called conformational mobility.
Rotational diffusion is the rotation of a molecule around its longitudinal (long) axis, since in a viscous medium “spindle” in the plane of the membrane it is easier to rotate around the longitudinal axis than around the transverse one.
Rotation around the transverse (short) axis is theoretically possible, but in this case the center of rotation inherent in the phospholipids is close to the surface. Rotation occurs as if about the "neck" of the molecule. In this area, the molecules are squeezed and movement is restricted. Tails in this case can make pendulum movements, but the center of rotation will be at the top. That is why the central part of the bilayer is more fluid than the regions of the fatty acid chains located closer to the polar head of the phospholipid molecule.
Intermolecular movement is the lateral diffusion of whole phospholipid molecules and, apparently, occurs through the exchange of two lipid molecules in places.
Fig. 1-4. The formation of lipids in the bilayer.
A is an example of a fragment of one of the bilayer leaves formed by the molecules (from left to right) phosphatidyl inositol, phosphatidylserine, phosphatidyl inositol, phosphatidylcholine, cholesterol. B - variants of lipid mobility in the bilayer. Intermolecular movement occurs in the form of exchange of two lipid molecules in places, and intramolecular movement in the form of rotation of the molecule around its longitudinal (long) axis and rotation around the transverse (short) axis. Tails can make pendulum movements.
Comments
To leave a comment
Human physiology, hygiene and age physiology
Terms: Human physiology, hygiene and age physiology