Lecture
Potential - controlled Ca 2+ channels allow Ca 2+ ions to enter cells when the membrane is depolarized. The role of Ca 2+ channels is especially significant for the interconnection of electrical potentials arising on the cell membrane with physiological processes occurring inside the cell. An increase in the cytosolic concentration of Ca 2+ causes various cellular reactions, for example, contraction, secretion, release of mediator, and transcription processes.
The Ca 2+ channels are a complex of proteins formed from the α 1 subunit and the auxiliary α 2 δ, β, and γ subunits. The α 1 subunit forms a conductive pore, it contains a voltage sensor and a channel gate apparatus. The loop between the transmembrane segments S5 and S6 in each domain determines the selectivity and conductivity of the channel. The selective filter of the Ca 2+ channel must recognize the Ca 2+ ion already at the channel entrance. These events are quite rare compared to the inputs of Na + ions, the number of which in the extracellular medium is about 100 times greater. Although the Ca 2+ and Na + ions have an identical diameter (2 Ao), the channel can choose Ca 2+ more than Na + in a 1000: 1 ratio. No sieve can so effectively differentiate ions of identical size. Most likely, it is time contains a specific place,
which is capable of binding Ca 2+ at its very low concentrations in solution (10 -6 M), and binds all other physiological ions in much higher concentrations.
Ca 2+ channels have been classified based on two principles:
• chemical symbol of the main ion for which they are permeable (in this case, ions
Ca 2+ );
• the principle of the physiological regulation of the channel, bearing in mind that these channels are potential-controlled (voltage gated calcium channels) Ca 2+ channels, which made it possible to introduce the designation CaV (Fig. 1-11 B).
The first digit after the designation corresponds in the subunit to the number of the gene subfamily (from 1 to 3 at present), and the second digit, which is placed after the dot, corresponds to the order of opening the original isoform α1 subunit within this subfamily (from 1 to m). According to this nomenclature, the Ca V 1 subfamily (from Ca V 1.1 to Ca V 1.4) includes channels containing α 1S , α 1C , α 1D and α 1F , which are characterized by L-type calcium currents. The Ca V 2 subfamily (from Ca V 2.1 to Ca V 2.3) includes channels containing α 1Α , α 1Β and α 1Ε , which are characterized by P / Q-type, N-type and R-type calcium currents, respectively. The Ca V 3 subfamily (from Ca V 3.1 to Ca V 3.3) includes channels containing α 1G , α 1Η and α 1I , for which T-type calcium currents are characteristic.
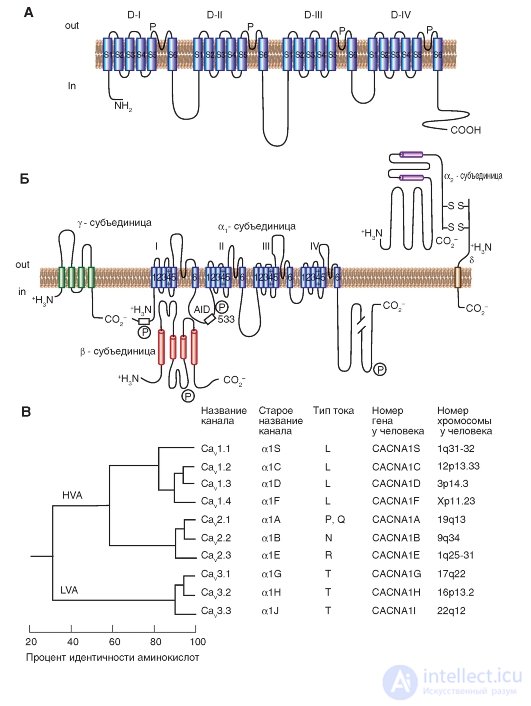
Fig. 1-11. Planometric model of the molecular organization of a potential-controlled Ca 2+ channel.
And - the main α 1 -subunit forming a time. The main structures of the α 1 -subunit are shown as transmembrane cylinders, representing α-helical segments. B - the model is made on the basis of experimental data studying the structure of Ca 2+ channels, consisting of five subunits. In addition to the α 1 subunit, there is a localized intracellular β subunit and an extracellular α 2 subunit linked by a disulfide bond to the δ subunit in the α 2 δ complex. The scale of the length of the lines approximately corresponds to the length of the polypeptide segments. The differences in the α 1 subunit between some Ca 2 + channels of the cell membranes are located in the NH 2 terminal part in the hydrophobic segments of DIS6 and DIVS3 and in the linker (cytoplasmic linking region) between domains I and II. AID (α 1 -interaction domain) is the primary binding region for all Ca V α 1 -subunits with Ca V β-subunits, named as α 1 -interactive domain. The symbol “P” designates phosphorylation sites for different protein kinases. B is the consistent similarity of α 1 -units of potential - controlled Ca 2+ channels. The phylogenetic tree of the primary sequences of Ca 2+ channels was demonstrated. As a result of the comparison of pairs of sequences, three families are clearly differentiated with interfamily sequences identified by more than 80% (Ca V 1, Ca V 2, Ca V 3). Further, sequence similarity was determined for each family, and these three sequences were compared with one another with interfamily sequences identified by 52% (Ca V 1 compared to Ca V 2) and 28% (Ca V 3 compared to Ca V 1 or with Ca v 2)
Ca 2+ channels were found in almost all cells. Ca 2+ -currents registered in different cell types have certain physiological and pharmacological properties. The original letter nomenclature was proposed based on the kinetics of Ca 2+ -currents. L-type (from “long-lasting” - long lasting) Ca 2+ -currents requires strong depolarization to activate, it is long lasting and is blocked by organic L-type antagonists of Ca 2+ channels, including dihydropyridines, phenylalkylamines and benzothiazepines. Ca 2+ L-type currents are central to muscle and endocrine cells, where they initiate contraction and secretion. N-type ( neither neither nor nor transient ” - neither L nor T), P / Q-type and R-type calcium currents also require strong depolarization to activate. They are relatively insensitive to L-type antagonists of Ca 2+ channels, but are blocked by specific polypeptide toxins from cochlea or spider venoms. These currents are mainly expressed in neurons, where they initiate neurotransmission for most fast synapses and also mediate the entry of Ca 2+ into cellular bodies and dendrites. The T-type (from “transient” - transient) of Ca 2+ -currents is activated by weak depolarization, and these currents are transient (transient). They are insensitive to organic antagonists and snake and spider toxins, which are used to determine the N- and P / Q Ca 2+ currents. Ca 2+ T-type currents are expressed in a large range of cell types, where they are involved in the development of an action potential and are important in cells and tissues with rhythmic activity.
The Ca 2+ channels, which have been characterized by biochemical methods, are a complex of proteins formed from four or five specific subunits encoded by a large family of genes.
At the molecular level, the Ca 2+ channel is composed of the formative pore of the transmembrane α 1 subunit and auxiliary α 2 δ, β, and γ subunits (Fig. 1-12 A). The α 2 subunit is located on the outer side of the membrane, the β subunit on the inner side of the membrane, and the δ and γ subunits are transmembrane structures. The structure of the Ca 2+ channel of the heart and smooth muscle is a structure with four subunits of the channel, and it resembles the Ca 2+ channels of the neuron. The auxiliary α 2 δ- and β-subunits increase the transport of Ca 2+ ions through the pore formed by the α 1 subunit and modulate the potential-dependent channel kinetics. The intracellular β-subunit and transmembrane disulfide-linked α 2 δ-subunit complex are components of most types of Ca 2+ channels. In addition to the role of the β-subunit in the transport of ions through the channel, it also plays a crucial role in electromechanical conjugation in skeletal muscle muscle fibers. The interactions of the α 2 δ- and β-subunit subunits are currently well studied. The presence of the γ subunit was also found in the skeletal muscle Ca 2+ channels, in cardiomyocytes and in brain neurons. Although these additional subunits modulate the properties of the ion channel, the pharmacological and electrophysiological features of the Ca 2+ channels are mainly related to the existence of α 1 subunits.
The β subunits of Ca 2+ channels are capable of forming heterogeneous complexes in vivo and in vitro. β-subunits and α 1 -subunit possess conservative areas of interaction, which contributes to the formation of heterogeneous channel complexes. The α 2 δ subunits modulate various α 1 subunits in vitro, and it is possible that this heterogeneity extends to in vivo situations . Such a diversity of subunit interactions would potentially increase the number of possible channel variants with differing biophysical and physiological properties, which may provide a variety of cellular responses.
The physiological model of the potential-controlled Na + channel is shown in the figure (Fig. 1-12 B).
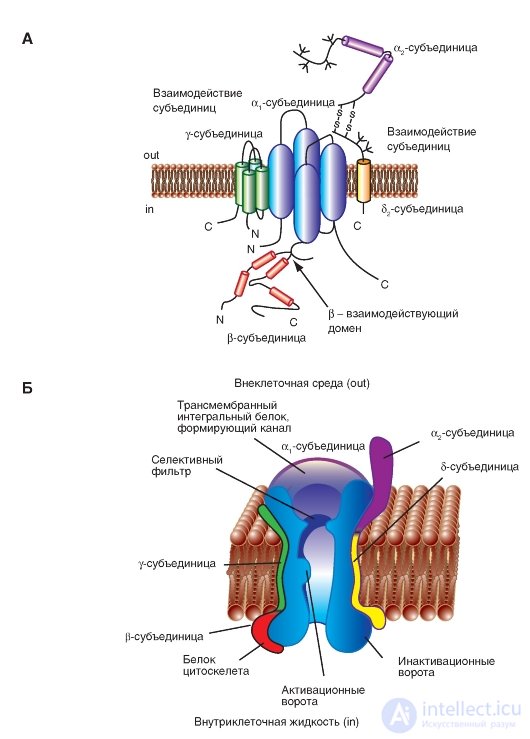
Fig. 1-12. Volumetric model of interactions of subunits of a potential-controlled Ca 2+ channel.
A - γ-subunit is depicted as a transmembrane protein with four segments with intracellular N- and C-termini. The first half of the γ-subunit interacts with the α 1 -subunit. The first extracellular loop contains charged residues and glycosylation sites. The interacting regions of the α 2 δ- and β-subunit (β-interacting domain) with the α 1 subunit were determined. B - physiological model of a potential-controlled Na + channel. The main α 1 -subunit forming the pore and additional α 2 -, δ-, γ- and β-subunits are shown.
Comments
To leave a comment
Human physiology, hygiene and age physiology
Terms: Human physiology, hygiene and age physiology