Lecture
Any electronic devices and systems from the point of view of providing electric energy can be represented in the form of the scheme shown in Figure 1.

Figure 1. Structural diagram of power electronic devices
This figure denotes: PIP - the primary power source - converts non-electric forms of energy into electrical energy; VIP - secondary power source - converts electrical energy to a form convenient for the consumer (load) and the actual load - radio electronic equipment (REA).
The primary power sources usually include:
These are dry electroplating cells, acid and alkaline batteries. The most common acid batteries (AB). Typical charge-discharge characteristics of one acid cell are shown in Figure 2.
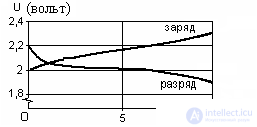
Figure 2. Charge – discharge characteristics of the acid element
During the discharge process, the voltage quickly decreases to 2 V, and then slowly drops to 1.8 V. Discharge below 1.8 V to one element is undesirable, since irreversible processes begin in it. The rated voltage is U = 2 V.
When charging an acid battery, its voltage quickly rises to 2.1 ... 2.15 V, and then slowly to 2.4 V, i.e. the recovery of the active mass of the battery is complete and the rapid release of oxygen and hydrogen begins, the charge is over. For sealed batteries, this is unacceptable, so they are placed in a special, durable "shell" that can withstand high pressure, add gas absorbers and strictly maintain the charge mode. The nominal capacity of a battery is the amount of electricity that a battery can give at a 10-hour discharge mode (C 10 ), constant current and temperature.
The operation of solar cells is based on a valve photoelectric effect in semiconductors (photo – emf at the p – n junction). Under the action of light, electrons transfer to a higher energy level, maintaining current in an external circuit. The spectral characteristics of some sources are shown in Figure 3.
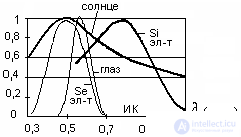
Figure 3. Spectral characteristics of sunlight and solar cells.
The maximum sensitivity of a silicon (Si) photocell is on the border of infrared (IR) radiation ( 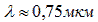 ). Selenium (Se) photocells are better matched in wavelength to sunlight and cover the visible part of the spectrum (0.4 µm is purple, 0.55 µm is green, 0.65 µm is red), which is not always convenient. Therefore, use of silicon, which is much more widely distributed on earth.
). Selenium (Se) photocells are better matched in wavelength to sunlight and cover the visible part of the spectrum (0.4 µm is purple, 0.55 µm is green, 0.65 µm is red), which is not always convenient. Therefore, use of silicon, which is much more widely distributed on earth.
It is known that the energy illumination of the Earth in the solar system is approximately 1 kW / m 2 , but this is at the equator. In middle latitudes, it is about 300 W / m 2 , but this is in summer, and in winter, approximately 80 W / m 2 . It is possible to extract this energy using silicon photocells with an efficiency of 12 ... 15% (theoretical efficiency is 22.5%, in arsenide – Galium photoelectric cells, theoretical efficiency is 33.3%). About 12 ... 15 photocells are required to produce 5V, 40mA, so there is no talk about high power for the industry. They are used on spacecraft with a solar cell surface of hundreds of square meters, as well as for charging batteries in places remote from human settlements.
There is an opinion that solar energy is exotic and its practical use is a matter of a distant future. The cost of solar cells is 2.5 ... 3 dollars / W, and the cost of electricity is 0.25 ... 0.5 dollars / kWh. When using solar panels, the problem of daily and seasonal accumulation of energy arises, which is solved using AB.
Fuel cells convert the energy of a chemical fuel into electrical energy, without a combustion reaction. The action of these elements is based on the electrochemical oxidation of hydrocarbon fuels (hydrogen, propane, methane, kerosene) in an oxidizing medium. In other words, Fuel cells are "inexhaustible batteries" to which fuel and oxidizer (air) are continuously supplied.
The following main types of fuel cells are distinguished:
Fuel cells have different operating temperatures and each has its own scope.
Since the voltage and current of a single fuel cell are low 0.6 ... 0.75 V at a current density of up to 500 mA / cm 2 , the fuel cells are combined into batteries to obtain the desired characteristics. In order to continuously generate electricity, an oxidizer and fuel should be continuously supplied to the battery.
Fuel cells are distinguished by high reliability (there are no moving parts as in an internal combustion engine) and thermal stability, and the specific energy is twice as high as that of rechargeable batteries. For this reason, modern electric cars use exactly fuel cells.
The work of thermogenerators is based on the thermoelectric effect - heating the contact of two conductors or semiconductors, which leads to the appearance at their free (cold) ends of the EMF, called thermo-EMF. The value of this thermo-emf 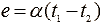 where
where 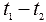 - the temperature difference between the cold and hot ends of the thermocouple,
- the temperature difference between the cold and hot ends of the thermocouple,  - thermo-emf coefficient, depending on the thermocouple material. Thermocouples are connected in series in the battery. Figure 4 shows the generalized thermopile scheme, and Figure 5 shows the temperature dependence of the thermo-emf of some thermocouples.
- thermo-emf coefficient, depending on the thermocouple material. Thermocouples are connected in series in the battery. Figure 4 shows the generalized thermopile scheme, and Figure 5 shows the temperature dependence of the thermo-emf of some thermocouples.
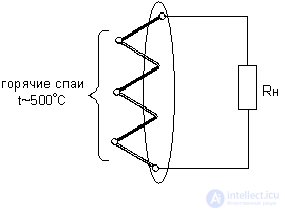
Figure 4. Generalized thermopile pattern
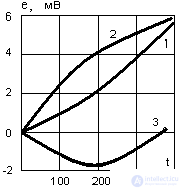
Figure 5. Dependence of thermo – emf of some thermocouples on temperature
This figure shows the value of thermo-emf thermocouples: 1 - Platinum and copper; 2 - Platinum and iron; 3 - Copper and iron. The dependences of thermo-emf shown in Figure 5 show that thermo-emf values are rather small, and creating a large temperature difference for metals is problematic because of their high thermal conductivity, therefore, semiconductors with electromotive forces of about 1 mV / ° C are used more often. Modern thermogenerators produce voltages up to 150 V and currents up to 500 A with an overall efficiency of about 10 ... 12%.

Figure 6. The appearance of thermopile
The principle of construction of atomic batteries is known from the course of general physics. One of the electrodes is a radioactive isotope, the second electrode is a metal sheath. Under the action of radiation on the electrodes, a potential difference of several kilovolts is created at a current of one milliampere. The service life of atomic elements is several years. At present, low-voltage atomic batteries operating on the principle of photovoltaic cells have been created, and their radiation does not exceed the level of the general background.
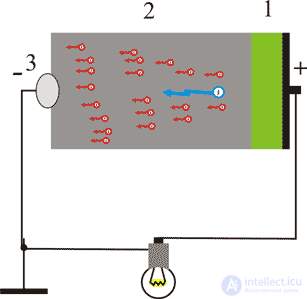
Figure 7. Low-voltage atomic battery: 1 - radioactive isotope; 2 - semiconductor; 3 - negative electrode; 4 - load, energy consumer
Consider the principle of operation of a low-voltage atomic battery. A layer of radioactive substance emitted by this layer is deposited on the surface of a semiconductor, the flow of beta particles bombards the semiconductor atoms, knocking out a very large number of slow electrons from it. Since knocked electrons can only move in one direction, they accumulate on the metal side of the semiconductor and forming Schottky contact with a semiconductor, which has one-sided conductivity. A potential difference arises between the collector and the semiconductor. To increase battery efficiency, instead of a pure semiconductor, the pn junction is often used as a contact with one-way conductivity. There are also batteries using the effect of thermionic emission, the so-called thermionic generators, to generate electrons. The principle of operation of such batteries is similar to the operation of high-voltage atomic batteries described above. In these batteries, isotopes are used, in which nuclear reactions lead to the heating of the cathode. A hot cathode emits slow electrons, which, reaching the anode, charge it negatively, while the cathode charges positively. One of the good reasons for using these energy sources is a number of advantages over other energy sources (practical non-servicing, compactness, etc.), and decisive the basis was the enormous energy intensity of the isotopes. Practically, in terms of mass and volume energy intensity, the decomposition of the isotopes used is inferior only to nuclear fission of uranium, plutonium, and others by a factor of 4–50, and exceeds chemical sources of energy (batteries, fuel cells, etc.) by tens and hundreds of thousands of times.
Figure 8. Appearance of a miniature nuclear battery
Most modern nuclear batteries use semiconductors to collect particles. Alas, over time, the "trap" becomes useless. Scientists from the University of Missouri replaced the solid semiconductor liquid, which allowed not only to make the battery miniature, but also durable. Its appearance is shown in Figure 9.
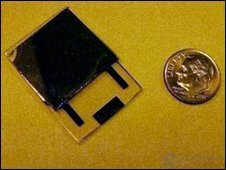
Figure 9. Appearance of a miniature nuclear battery
Transform the mechanical energy of motion (translational or rotational) into electrical energy and vice versa. Available for a large range of currents and voltages. Electrical machines are divided into electric machines, AC and DC. With the same power, AC electric machines have 1.5 ... 2 times better mass-volume indicators than DC machines. Therefore, 98% of the world's electricity is generated by electric AC machines. Their disadvantages are the presence of acoustic noise, and the presence of moving parts determines the reliability of the power supply system. But the inertia of electric machines makes short-term dips in the network voltage impossible, which has a positive effect on the quality of power supply.
Depending on how the alternator rotates distinguish:

Figure 10. The appearance of the diesel generator set
Diesel generator sets usually have more power and are used to power large telecommunications enterprises, which include more power consumption *** electronic equipment.

Figure 11. The appearance of the gas generator
Petrol generators can be used for guaranteed power supply of base stations of cellular communication systems, repeaters, repair services or car repair shops.
Literature:
Comments
To leave a comment
Power supplies for electronic equipment
Terms: Power supplies for electronic equipment