Lecture
The chemical source of the current (abbr. HIT ) is the source of emf, in which the energy of chemical reactions proceeding in it is directly converted into electrical energy.
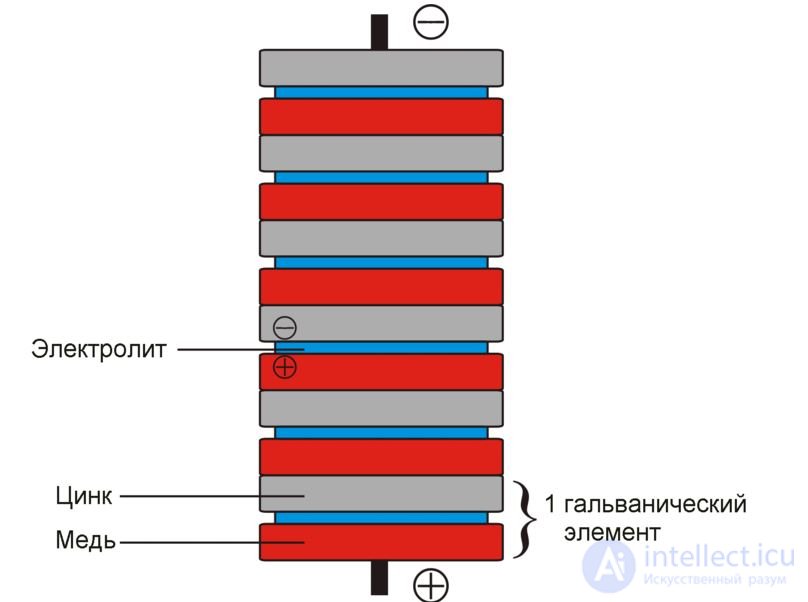
The first chemical source of current was invented by the Italian scientist Alessandro Volta in 1800. It was the "Volta element" - a vessel with salt water with zinc and copper plates connected to it, connected by a wire. Then the scientist assembled a battery of these elements, which was later called the "volt column." This invention was later used by other scientists in their research. For example, in 1802, Russian Academician V.V. Petrov designed a volt pole of 2,100 elements to produce an electric arc. In 1836, English chemist John Daniel improved the element of Volta by placing zinc and copper electrodes in a solution of sulfuric acid. This design has become known as the "element of Daniel."
In 1859, the French physicist Gaston Plante invented a lead-acid battery by placing a thin lead plate rolled into a roll in sulfuric acid. This type of element is still used in car batteries.
In 1865, the French chemist J. Leclanche proposed his own galvanic element (Leclanche element) consisting of a zinc cup filled with an aqueous solution of ammonium chloride or another salt chloride, in which the manganese (IV) MnO 2 sinter was placed with a carbon lead current. A modification of this design is still used in salt batteries for various household devices.
In 1890 in New York, Conrad Hubert, an immigrant from Russia, created the first pocket-sized electric torch. And in 1896, the National Carbon company began mass production of the world's first dry elements Leclanche “Columbia”.
The longest electroplating cell is a silver-zinc battery manufactured in London in 1840. Connected to two such series-connected batteries, the bell still works in the Clarendon laboratory at Oxford. [one]
The basis of chemical current sources are two electrodes (a negatively charged anode containing a reducing agent, and a positively charged cathode containing an oxidizing agent) in contact with the electrolyte. A potential difference is established between the electrodes — the electromotive force corresponding to the free energy of the redox reaction. The action of chemical current sources is based on the flow of spatially separated processes when the external circuit is closed: the reducing agent oxidizes at the negative anode, the resulting free electrons pass through the external circuit to the positive cathode, creating a discharge current where they participate in the oxidizer reduction reaction. Thus, the flow of negatively charged electrons through an external circuit goes from the anode to the cathode, that is, from the negative electrode (negative pole of the chemical current source) to the positive one. This corresponds to the flow of electric current in the direction from the positive pole to the negative, since the direction of the current coincides with the direction of movement of the positive charges in the conductor.
In modern chemical current sources are used:
When it is possible or impossible to reuse chemical current sources are divided into:
It should be noted that the division of cells into galvanic cells and batteries is somewhat conditional, since some galvanic cells, such as alkaline batteries, are rechargeable, but the efficiency of this process is extremely low.
By the type of electrolyte used, chemical current sources are divided into acidic (for example, lead-acid battery, lead-fluoric cell), alkaline (for example, mercury-zinc element, mercury-cadmium element, nickel-zinc battery, nickel-cadmium battery) and salt (for example , manganese-magnesium element, zinc-chlorine battery).
A galvanic cell is a chemical source of electrical current, named after Luigi Galvani. The principle of operation of a galvanic cell is based on the interaction of two metals through an electrolyte, leading to the appearance of an electric current in a closed circuit.
See also Category: Electroplating.
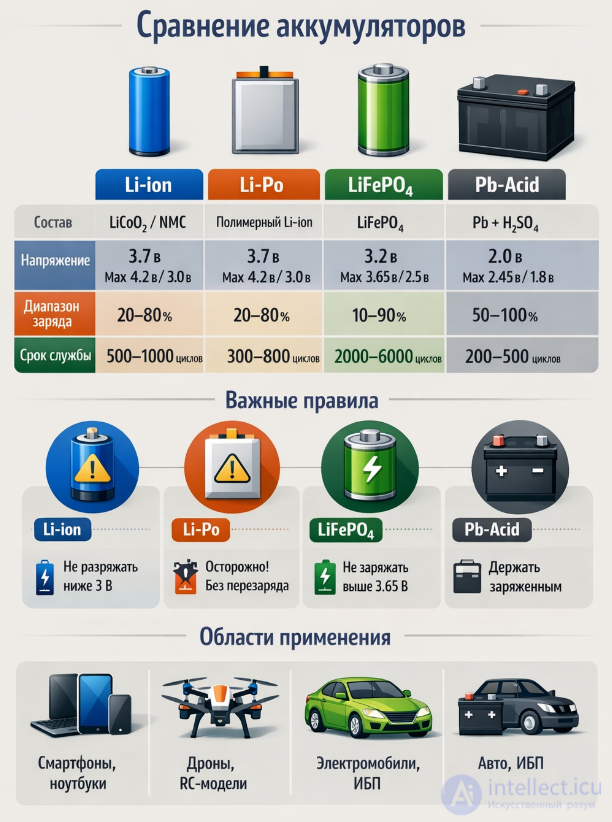
| Type of | Cathode | Electrolyte | Anode | Voltage, AT |
|---|---|---|---|---|
| Manganese-zinc element | MnO 2 | KOH | Zn | 1.56 |
| Manganese-tin element | MnO 2 | KOH | Sn | 1.65 |
| Manganese-magnesium element | MnO 2 | MgBr 2 | Mg | 2.00 |
| Lead-zinc element | PbO 2 | H 2 SO 4 | Zn | 2.55 |
| Lead-cadmium element | PbO 2 | H 2 SO 4 | Cd | 2.42 |
| Lead-chlorine element | PbO 2 | HClO 4 | Pb | 1.92 |
| Mercury-zinc element | Hgo | KOH | Zn | 1.36 |
| Mercury-cadmium element | HgO 2 | KOH | Cd | 1.92 |
| Oxide-mercury-tin element | HgO 2 | KOH | Sn | 1.30 |
| Chrome Zinc | K 2 Cr 2 O 7 | H 2 SO 4 | Zn | 1.8—1.9 |
Other types:
An electric accumulator is a chemical source of reusable current (that is, unlike a galvanic cell, chemical reactions that are directly converted into electrical energy are repeatedly reversible). Electric batteries are used for energy storage and autonomous power supply of various devices.
See also Category: Batteries.
A fuel cell is an electrochemical device that is similar to a galvanic cell, but differs from it in that substances for an electrochemical reaction are fed into it from the outside, in contrast to the limited amount of energy stored in a galvanic cell or battery.
See also Category: Fuel cells.
A battery (automobile ordinary) is a device capable of both absorbing (charging) and giving (discharging) electrical energy as a result of the reversible chemical reaction occurring inside the device. A conventional car battery is not a source of current, but a source of electrical energy, and more specifically, a source of voltage. Since only the voltage source is able to maintain the specified value of the potential difference when the load resistance changes.
Comments
To leave a comment
Power supplies for electronic equipment
Terms: Power supplies for electronic equipment