Lecture

A nickel-metal hydride battery (Ni-MH) is a secondary chemical current source, in which the anode is a hydrogen metal hydride electrode (usually nickel-lanthanum or nickel-lithium hydride), potassium hydroxide electrolyte, and oxide-nickel cathode.
Research into the technology of manufacturing NiMH batteries began in the 1970s and was undertaken as an attempt to overcome the shortcomings of nickel-cadmium batteries. However, the metal hydride compounds used at that time were unstable and the required characteristics were not achieved. As a result, the development process for NiMH batteries has stalled. New metal-hydride compounds, sufficiently stable for use in batteries, were developed in 1980. Since the late 1980s, NiMH batteries have been continuously improved, mainly in terms of the density of stored energy. Their developers noted that for NiMH technologies there is the potential to achieve even higher energy densities.
In nickel-metal hydride batteries of the “Krona” type, as a rule, with an initial voltage of 8.4 V, the voltage gradually decreases to 7.2 V, and then, when the battery energy is exhausted, the voltage decreases rapidly. This type of battery is designed to replace nickel-cadmium batteries. Nickel-metal hydride batteries have about 20% more capacity with the same dimensions, but less service life - from 200 to 500 charge / discharge cycles. Self-discharge is about 1.5-2 times higher than that of nickel-cadmium batteries.
NiMH batteries are practically free from the "memory effect". This means that you can charge an incompletely discharged battery if it has not been stored for more than a few days in this condition. If the battery was partially discharged, and then not used for a long time (more than 30 days), then it must be discharged before charging.
Ecologically safe.
The most favorable mode of operation: charge a small current, 0.1 nominal capacity, charge time - 15-16 hours (typical recommendation of the manufacturer).
On batteries affects: [1]
1) Charge:
Choose an intelligent charger that is capable under certain conditions (for example, when the battery is charged or recharged at negative voltage, or overheats) to turn off automatically. Usually a charge with a lower speed allows you to extend battery life compared to using a quick charger.
2) Discharge:
- The battery life is significantly affected by the depth of discharge (DOD). The higher the DOD, the shorter the battery life and vice versa. Therefore, deep discharge of batteries to very low voltage should be avoided. Depending on the discharge voltage, the value from 0.8 V to 1.0 V can be considered as the permissible voltage at the battery terminals.
- Discharge of batteries at high temperatures will shorten their life.
- If the electronic device does not completely block the battery charge (for example, consumes current in the standby mode), finding the batteries inside the device for a long time can lead to their deep discharge.
- The use of a combination of old and new batteries or batteries of different capacity, chemical composition and charge level can lead to their deep discharge or even charge with reverse polarity.
3) Storage:
- Long-term storage of batteries in places with high temperatures reduces their service life.
- Do not forget to remove the batteries from the charger after charging.
Nickel-metal hydride batteries with low self-discharge (the low self-discharge nickel-metal hydride battery, LSD NiMH), were first introduced in November 2005 by Sanyo under the brand name Eneloop. Later, many global manufacturers presented their LSD NiMH batteries.
This type of battery has a reduced self-discharge, and therefore has a longer shelf life compared to conventional NiMH. Batteries are sold as “ready-to-use” or “pre-charged” and are positioned as a replacement for alkaline batteries.
Compared to conventional NiMH, LSD NiMH batteries are most useful when it can take more than three weeks between charging and using the battery. Conventional NiMH batteries lose up to 10% of the charge capacity during the first 24 hours after the charge, then the self-discharge current stabilizes at up to 0.5% of the capacity per day. For LSD NiMH, this parameter is usually in the range from 0.04% to 0.1% of the capacity per day. Manufacturers claim that by improving the electrolyte and electrode, the following advantages of LSD NiMH with respect to classical technology were achieved:
Incomplete list of long-term storage batteries (with low self-discharge):
Other Benefits of Low Self Discharge NiMH Batteries (LSD NiMH) Low self discharge nickel metal hydride batteries typically have significantly lower internal resistance than regular NiMH batteries. This has a very positive effect on high current consumption applications:
Charging is performed by an electric current with a voltage on the element up to 1.4 - 1.6 V. The voltage on a fully charged element without a load is 1.4 V. The voltage at a load varies from 1.4 to 0.9 V. The voltage without load is completely a discharged battery is 1.0 - 1.1 V (further discharging may damage the cell). To charge the battery, direct or pulsed current with short-term negative pulses is used (to prevent the “memory” effect, the method of charging batteries with an alternating asymmetric current).
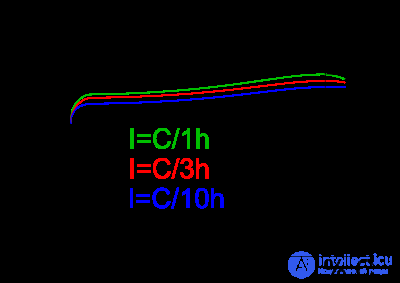
One of the methods for determining the end of charge is the -ΔV method. The image shows a graph of the voltage on the element during charge. The charger charges the battery with constant current. After the battery is fully charged, the voltage on it begins to fall. The effect is observed only at sufficiently high charging currents (0.5S..1C). The charger should detect this drop and turn off charging.
There is also the so-called "inflexion" - a method for determining the end of fast charging. The essence of the method lies in the fact that it is not the maximum voltage across the battery that is analyzed, but the maximum of the time derivative of the voltage. That is, fast charging will stop at the moment when the rate of voltage rise will be maximum. This allows you to complete the fast charge phase earlier, when the battery temperature has not yet had time to rise significantly. However, the method requires measuring the voltage with greater accuracy and some mathematical calculations (calculating the derivative and digital filtering of the obtained value).
When charging an element with a direct current, most of the electrical energy is converted into chemical energy. When the battery is fully charged, the supplied electrical energy will be converted to heat. With a sufficiently large charging current, you can determine the end of the charge by a sharp increase in the element temperature by installing a battery temperature sensor. Maximum permissible battery temperature is +60 ° C.
The following formula is used to calculate the battery charge time: t = 1.3 * (battery capacity / charge current) [2]

Replacement of a standard galvanic cell, electric cars, defibrillators, rocket and space equipment, autonomous power supply systems, radio equipment, lighting equipment.
When using NiMH batteries, it is not always necessary to chase after a large capacity. The more capacious a battery is, the higher (all other things being equal) its self-discharge current. For example, consider the battery capacity of 2500 mAh and 1900 mAh. Fully charged and unused for, for example, a month, batteries lose some of their electrical capacity due to self-discharge. A more capacious battery will lose its charge much faster than a less capacious one. Thus, after a month, for example, the batteries will have approximately equal charge, and after an even longer time, the initially more capacious battery will contain a smaller charge.
From a practical point of view, high-capacity batteries (1500–3000 mAh for AA-batteries) it makes sense to use in devices with high energy consumption for a short time and without prior storage. For example:
Batteries of the same low capacity (300-1000 mAh for AA-batteries) are more suitable for the following cases:
Nickel-metal hydride batteries are manufactured by different companies, including:
Comments
To leave a comment
Power supplies for electronic equipment
Terms: Power supplies for electronic equipment