Lecture
1. INTRODUCTION
Sensors (sensors) allow you to receive, register, process and transmit information about the status of various systems. This may be information about the physical structure, chemical composition, shape, position and dynamics of the system under study. There are various types of sensors. The principles of their action are based on certain physical or chemical phenomena and properties. Examples are well-known temperature sensors, radars, echo sounders, radiation level sensors, pressure sensors, hygrometers, etc. [ 1 - 18 ].
Advances in areas such as laser physics, solid state physics, microelectronics, microprocessor technology, Internet technologies, materials science, quantum electronics, and integrated optics have led to the development of a new direction in the development of sensors - the creation of chemical sensors [ 4 ].
One of the most promising types of chemical sensors in our opinion are optical chemical sensors [ 3 ]. Integrated-optical chemical sensors are very promising among them [ 9 - 12 , 17 , 18 ]. The principle of operation of an integrated optical chemical sensor, for example, an absorption type, is based on recording changes in the intensity of laser radiation interacting with the gaseous (gas, steam) or liquid medium at certain wavelengths characteristic of this medium [ 9 - 12 , 17 , 18 ] .
The relevance and practical significance of this review is due to the possibility of detecting a number of gases critical for human safety using various chemical sensors. The solution to this problem is of priority importance for the electronic industry, the chemical industry, the oil and gas industry (mining, transportation, storage), ecology, medicine, military technology, etc.
2. SENSOR SYSTEMATICS
When organizing sensors, they often consider the principle of their action, which may be due to physical or chemical phenomena and properties. In fig. 1 shows a generalized functional diagram of a measurement using a chemical sensor.
There are many phenomena, effects, and types of energy conversion that can be used to build sensors [ 1 - 21 ]. The Table gives examples of such phenomena and effects (see, for example, [ 1 - 4 ]).
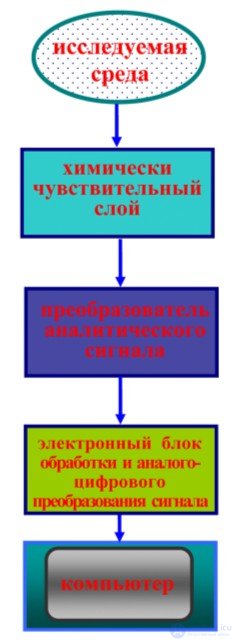
Fig. 1. Functional diagram of the measurement using a chemical sensor.
Table.
|
Effect, phenomenon, property |
The physical essence of the transformation |
|
Thermal conductivity (thermal energy) |
The transfer of heat inside a physical object from an area with a higher to an area with a lower temperature |
|
Thermal radiation (thermal energy |
Optical radiation with increasing temperature of a physical object |
|
Seebeck effect (temperature |
The emergence of EMF in a circuit with bimetallic compounds at different temperatures of junctions |
|
Pyroelectric effect (temperature |
The appearance of electric charges on the faces of some crystals with increasing temperature |
|
The effect of photoconductivity (light |
Change in electrical resistance of a semiconductor when it is irradiated with light |
|
Faraday effect (light and magnetism |
Rotation of the plane of polarization of a linearly polarized light beam passing through paramagnetic substance |
|
Piezoelectric effect (pressure |
The occurrence of a potential difference on the faces of a ferroelectric under pressure |
|
Doppler effect (sound, light |
Frequency change during mutual movement of objects compared to the frequency when these objects are motionless |
|
Chemical properties (information on chemical bonds |
A biochemical converter converts information about chemical bonds into a physical or chemical property or signal |
Without claiming to be comprehensive, we give some useful classification of sensors [ 1 - 21 ].
The energy properties of the input values of the sensors make it possible to divide them according to the type of input values into active and passive . In active sensors, the input quantities are of an energetic nature (voltage, force, etc.), while in passive sensors, the input quantities are non-energetic (electrical capacitance, resistance, etc.).
According to the number of perceived and transformed quantities , one-dimensional sensors that operate with one value and n- dimensional ( multidimensional ) sensors that sense several ( n ) input quantities can be distinguished . At the same time, multidimensional sensors can have common elements and, therefore, be simpler than a set of one-dimensional sensors that perceive the same amount.
According to the number of performed (measuring) functions, one- function and multi-function sensors can be distinguished. Multifunctional can, in addition to the main function (perception of the value and the formation of the measuring signal) perform a number of additional functions.
Multifunction sensors are sometimes also called smart sensors. Such sensors, in principle, include analog and digital sensors with summation of signals, with adaptive adaptive operating modes and parameters, with analog-to-digital conversion, metrological maintenance, and sensors with built-in microprocessors.
Additional functions of multifunction sensors include the following:
data processing and filtering operations;
error correction;
signal storage;
conversion of the "field" of signals into an image;
protection against interference;
and etc.
According to the number of energy and substance conversions, the sensors can be divided into single-stage and multi-stage .
According to the manufacturing technology, the sensors can be divided into elementary , made from a set of separate elements, and integral , in which all the sensor constituent elements are made simultaneously using integrated technology.
Biological sensors are especially distinguished, in which the receptor part of the biological sense organs, enzymes and other substances, as well as the electronic part, which forms the measuring signals, are used as sensitive elements.
According to the interaction with information sources, the sensors are divided into contact and non-contact (remote action).
According to the type of measuring signals, the sensors are divided into analog and digital . To analyze the operation of analog and digital sensors, the appropriate mathematical apparatus should be used.
Currently, there is a tendency to increase the number and complexity of the functions performed by sensors. This is especially true for integrated sensors, which may include additional devices. Such sensors can serve as the basis for the creation of measuring systems that allow the collection, processing, storage and distribution of information (see, for example, [2, 12]).
The following basic requirements are presented to modern sensors:
high quality characteristics: sensitivity, accuracy, linearity, reproducibility of readings, response speed, interchangeability, the absence of hysteresis and a large signal-to-noise ratio;
high reliability: long service life, resistance to the external environment, failure-free operation;
manufacturability: small dimensions and weight, simplicity of design, integrated design, low cost.
In the future, we will focus on various types of chemical sensors. Attention to chemical sensors is dictated by a number of reasons, among which safety problems are now the most relevant.
3. CHEMICAL SENSORS
3.1. Some stages of the development of chemical sensors
To date, a huge number of a wide variety of chemical sensors have been developed.  . The beginning of the history of chemical sensors can be considered the end of the XIX - beginning of the XX century. At this time, a prototype of a katharometer ( 1880 ) appeared, which was used to determine the hydrogen content in water vapor; Kohlrausch’s two-electrode cell ( 1885 ), Nernst metal electrodes ( 1888 ), and Kremer’s glass electrode ( 1906 ). In the late XIX - early XX centuries. sensors (the word "sensor" from the English word sense - sense, sensation) meant portable devices for determining the chemical composition of the medium. A typical sensor design included a sensing element and a transducer [4].
. The beginning of the history of chemical sensors can be considered the end of the XIX - beginning of the XX century. At this time, a prototype of a katharometer ( 1880 ) appeared, which was used to determine the hydrogen content in water vapor; Kohlrausch’s two-electrode cell ( 1885 ), Nernst metal electrodes ( 1888 ), and Kremer’s glass electrode ( 1906 ). In the late XIX - early XX centuries. sensors (the word "sensor" from the English word sense - sense, sensation) meant portable devices for determining the chemical composition of the medium. A typical sensor design included a sensing element and a transducer [4].
At that time, the standard chemical analysis procedure was a multi-stage process based on chemical reactions. Thus, chemical analysis was then fully "chemical." And already in the first sensors physical and physicochemical processes were used.
The next stage in the development of chemical sensors is associated with the advent of flow analysis methods. In the 50s of the XX century. analytical instrumentation has reached such a level that it has become possible to create flow-through analysis methods. In 1952 Martin and James proposed a gas chromatograph. In all cases, there was an urgent need for detectors - devices that would automatically determine the concentration of a substance in a gas or liquid stream.
The next important moment in the development of sensory analysis can be considered Bergfeld’s proposal to combine a sensitive membrane with a gate of a field effect transistor. This proposal led to the appearance of an ion-selective field effect transistor. In addition, there are prospects that the planar technology developed in microelectronics will lead to the creation and mass production of low-cost sensors.
The small size and relatively small size of the sensors allows you to create their sets in a small volume. So, on one semiconductor crystal, you can place several sensitive elements or in a small volume several independent sensors. Thus, it became possible to create a “laboratory on a chip” equipped with a microprocessor for processing analysis results (see, for example, [4]).
3.2. The device and principles of operation of chemical sensors
Chemical sensors are sensors in which two types of transducers - chemical and physical - are in close contact with each other.
A chemical converter consists of a layer of sensitive material, which forms a selective response to a determined component: it is able to reflect the presence of a determined component and a change in its content.
A physical transducer - a transducer - converts the energy that occurs during the reaction of a selective layer with a determined component into an electrical or light signal. This signal is then measured using a photosensitive and / or electronic device.
Chemical sensors can work on the principles of chemical reactions and on physical principles. In the first case, the analytical signal is due to the chemical interaction of the component being determined with the sensitive layer, which acts as a converter. In the second case, a physical parameter is measured (absorption or reflection coefficient of light, mass, conductivity, etc.).
To increase the selectivity of the input device, membranes are placed in front of the chemically sensitive layer, which selectively pass particles of the component being determined (ion-exchange, hydrophobic, and other films). In this case, the substance to be determined diffuses through a semipermeable membrane to a thin layer of a selective layer in which an analytical signal is formed on the component.
Based on chemical sensors, sensory analyzers are being developed, which are devices for determining a substance in a given range of its concentrations. Note that biosensors also belong to chemical sensors.
Depending on the nature of the response (primary signal) that occurs in the sensitive layer of chemical sensors, they are divided into the following types:
electrochemical (potentiometric, coulometric, etc.);
electrical (semiconductor based on metal oxides, etc.);
magnetic (Hall sensors, magnetoresistive semiconductor elements, etc.);
thermometric;
optical (luminescent, spectrophotometric, etc.);
biosensors (based on various biological material: enzymes, tissues, bacteria, antigens, receptors, etc.);
and etc.
Let us dwell briefly on the work of some types of electrochemical sensors, thermistor sensors, biosensors, and integrated optical chemical sensors.
3.3. Electrochemical sensors
In the electrochemical sensor, the detected component reacts with the sensitive layer directly on the electrode or in the volume of the solution layer near the electrode. Among the electrochemical sensors, the following are distinguished:
- potentiometric,
- amperometric,
- conductometric,
- coulometric.
Potentiometric sensors are based on ion-selective electrodes, which give a selective response to the presence of detected ions or molecules of substances in solutions. The analytical signal in them is the potential that forms on the surface of a solid material placed in a solution containing ions that can exchange with the surface. The magnitude of the potential is related to the number of ions in the solution. It is not possible to measure the surface potential directly, but it can be measured using an appropriate electrochemical cell. This is the essence of the potentiometric method.
It should be noted that zero current is needed to measure the cell potential. In practice, such a condition is unattainable, since the process of measuring the potential itself implies the presence of a small current. But since the current here is in the microampere range, it slightly distorts the equilibrium potential on the surface. Thus, the assumption that the potential is measured essentially under zero current conditions is quite correct.
There are various types of ion selective electrodes. Their classification is based on the difference in selective chemical reactions leading to the formation of an interfacial potential. Specific recognition by a potentiometric chemical sensor is achieved through a chemical reaction on the surface of the sensor. Thus, the surface of the electrode must contain a reagent that chemically and reversibly interacts with the analyte. This is achieved through the use of ion-selective membranes, which represent the surface of the sensor. Potentiometric sensors use four types of membranes:
- Glass membranes. Such membranes selective with respect to ions such as H + , Na + and NH 4 .
- Membranes from poorly soluble inorganic salts. TO membranes of this type include monocrystalline organic salts, for example LaF 3 , or disks from a compressed powder of inorganic salt or a mixture of salts, for example, Ag 2 S / AgCl . These membranes are selective with respect to ions such as F - , S 2 - and C l - .
- Polymeric membranes with immobilized ionophore. In these membranes, ion-selective complexing compounds or ion exchangers are immobilized in a polymer matrix, for example, in polyvinyl chloride.
- Membranes with enzymes immobilized in a gel or chemically bound to a gel. In membranes of this type, highly specific reactions catalyzed by enzymes are used. The enzyme is contained within the matrix or chemically grafted onto a solid surface.

Thanks to advances in microelectronics, ion-selective field effect transistors have been developed . They are a modified insulated gate field effect transistor.
The main part of the ion-selective field effect transistor is the p- type semiconductor, in which there are two sections, which are the n- type semiconductors , called, respectively, the source and drain (Fig. 2). A metal oxide insulator is applied to the surface of the semiconductor, onto which an ion-selective membrane is then applied instead of the gate metal of the field-effect transistor. The current passing between the source and drain is determined by the input voltage.
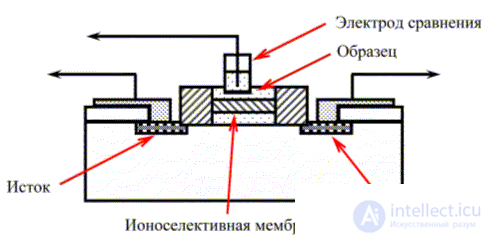
Fig. 2. Ion-selective field effect transistor.
The test solution with the reference electrode immersed in it is in contact with the ion-selective membrane, which leads to the appearance of a potential on the membrane surface, which is the input potential that controls the current between the drain and the source. The current strength depends on the membrane potential, which, thus, depends on the activity of the determined ions in the test solution. Such devices are extremely small (<1 mm 2 ) and are widely used to determine a variety of substances.
In oltammetry . This method consists in measuring the current strength in an electrochemical cell as a function of the applied potential.
Many substances are oxidized or reduced at a certain potential, which is characteristic of a given substance. If the potential is fixed at a value corresponding to the oxidation or reduction of the analyte, then the current strength is directly related to its concentration. The action of amperometric electrochemical sensors is based on this principle.
For example, an oxygen amperometric sensor is used to measure the concentration of oxygen dissolved in water. This sensor has a gold or platinum cathode, separated from the silver anode by a plastic sheath. A gas permeable membrane, which is located on the outside of the lower surface of the electrode, allows small molecules to pass through. When the sensor is immersed in the studied water sample, oxygen molecules diffuse into a thin electrolyte film in contact with the electrodes. A potential of -800 mV relative to the silver anode is maintained at the cathode, and molecular oxygen is reduced in accordance with the equation:
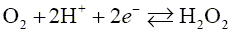 (1)
(1)
The current passing through the cell is measured and the concentration of dissolved oxygen is determined by its magnitude. Such a sensor must be calibrated using standard solutions with a known concentration of dissolved oxygen.
The selectivity of amperometric chemical sensors is determined mainly by the nature of the material of the electrode surface, and, consequently, by the magnitude of the potential at which electrochemical reactions occur with the participation of the analyzed component.
To increase the selectivity of the response, the surface of chemical sensors is modified using special compounds that carry electron transfer between the electrode and the detected component. The operation of fixing the carrier modifier on the surface of a chemical sensor is called immobilization. In this case, the modifier ceases to be mobile, is not washed out by the analyzed solution and can work in a fluid stream. Modification of electrodes for chemical sensors lengthens their service life.
The sensitivity of amperometric electrochemical sensors is usually higher than potentiometric.
Conductivity sensors . Their action is based on measuring the electrical conductivity of solutions. Such electrochemical sensors are used, in particular, to determine the concentration of CO 2 in air. In this case, the electrical conductivity of an aqueous solution of carbon dioxide is measured, in which, as a rule, as a result of its dissociation, H + ions are formed in amounts depending on the partial pressure of CO 2 in the air. The difference in electrical conductivity between the "idle" solution (without CO 2 ) and the analyzed (with CO 2 ) is recorded as an analytical signal.
Coulometric sensors . The work of this type of electrochemical sensors is based on the dependence of the current flowing through the electrochemical cell at a controlled flow rate of the analyzed gas supplying the cathode, on the oxygen concentration (provided that oxygen is almost completely pumped out of the stream). They are less known, but in some cases the accuracy of their measurement is higher than other types of electrochemical chemical sensors.
In conclusion of this section, it should be noted that several types of potentiometric and amperometric ammonia sensors based on microorganisms have been developed [21]. A typical ammonia microbial sensor consists of immobilized bacteria, a gas permeable Teflon membrane, and an oxygen electrode. The relationship between the decrease in current and the concentration of ammonia is linear up to a concentration of 42 mg / L. The lower limit of the determined concentrations was 0.1 mg / L. The sensitivity of the microbial sensor was approximately equal to the sensitivity of the glass electrode. The sensor did not respond to volatile compounds such as acetic acid, ethanol and amines, or non-volatile nutrients such as glucose, amino acids and metal ions. The output current of the sensor was stable for more than 10 days with 200 analyzes.
3.4. Thermistor sensors
A thermistor is a device for measuring temperature changes. The basis of its action is the phenomenon of a decrease in the electrical resistance (approximately 4-7% / ° C) of metal oxides (BaO / CaO, transition metal oxide) alloyed at high temperature.
Thermistors are useful for measuring temperatures with an accuracy of ± 0.005 ° C. They can be of different sizes and shapes, but the thermistor in the form of a ball covered with a glass protective layer is most convenient for the sensor.
Resistance and temperature are usually measured using the Winston bridge, which serves to measure resistance.
High sensitivity to small changes in temperature, which differ thermistors, can be used to determine the small amounts of heat that are released during the chemical reaction. This is how thermistors are used in microcalorimetry when chemical reactions are studied in the bulk phase of a solution. When applied to sensors, selectivity with respect to the substance to be determined is required, which is achieved as a result of a chemical reaction on or near the surface of the thermistor.
There are two main approaches to using thermistors in calorimetric sensors. In accordance with one, a thermistor is placed in a detector cell to measure temperature changes after the analyte solution is passed through a layer of immobilized enzyme. Although such a detector system can be adapted to detect several analytes, significant amounts of the enzyme are needed for this. The second approach is to immobilize the enzyme directly on the surface of the thermistor. In this case, the sensor can be miniature and can be placed in a flow-through analytical system. For example, consider two types of thermistor chemical sensors.
Catalytic gas sensors
Catalytic gas sensors are widely used to detect combustible gases (methane, ethane, propane, carbon monoxide and hydrogen) and vapors (gasoline, organic solvents) in the air.
The principle of their action is the controlled combustion of combustible gas in the air and the measurement of the amount of heat generated during this. In order to accelerate the response, catalysts are used. Thus, a catalytic gas sensor needs a heater to maintain a temperature sufficient to burn gas, an oxidation catalyst and a device for measuring the calorific value. Usually, a spiral of wire is used as a heater, and the temperature dependence of the resistance of this wire is used to measure the heat generated [ 2 , 4 ].
The first catalytic gas sensor used a platinum spiral, which was heated by passing current through it to the gas burning temperature on the platinum surface. Heat generation led to heating of the spiral and, consequently, to an increase in its resistance. The change in temperature was used to determine the amount of burned gas.
As a catalyst, platinum loses to other metals such as palladium and rhodium: when using platinum, much higher temperatures (1000 ° C) are needed, which leads to a significant loss of platinum and a decrease in wire thickness.
The need for other forms of catalytic gas sensors has led to the creation of pellistors. The pellistor is a gas sensor based on the same principle as the previous one, that is, it also uses a platinum spiral as a heating element and a resistance thermometer as a temperature sensor. The difference is that in this case, the quality of the catalyst uses palladium in the form of a fine powder, which allows to increase the surface area and significantly increase the efficiency of the catalyst. Thus, the oxidation catalyst in this sensor is much more efficient [ 4 ]. This allows the sensor to be used at temperatures of about 500 ° C, that is, to detect hydrocarbons such as methane.
The pellistor circuit is shown in Fig. 3. The platinum coil in this sensor is enclosed in a refractory ball about 1 mm in size . The surface of the ball is covered with a layer of finely divided palladium in a matrix of thorium oxide [ 4 ].
Electronics for measuring systems can operate in feedback mode. In this case, the current for heating the platinum wire is reduced in order to compensate for the temperature increase caused by combustion. In this case, the current strength 
 is a measurable parameter associated with a change in temperature caused by the burning of gas, and therefore with the amount of gas.
is a measurable parameter associated with a change in temperature caused by the burning of gas, and therefore with the amount of gas.
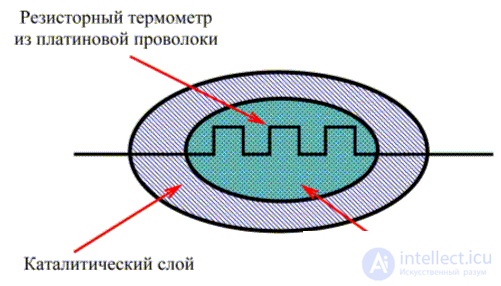
Fig. 3. The scheme of the pellistor .
The problem with the use of gas sensors is that they are exposed to the toxic effects of other gases, which leads to a loss of selectivity of the sensors. To solve this problem, pellistors and sensor systems with lower susceptibility to substances poisoning the sensors are being developed.
The pellistor design, in which a platinum coil surrounds a porous aluminum ball containing a large amount of finely ground catalyst, turned out to be the best solution. In this case, the available surface area of the catalyst increases significantly, but the mechanical strength of the pellistor decreases in comparison with the previous design.
All equipment related to gas thermistor sensors is quite simple and portable, therefore a pocket-sized device is usually used. For gas sensors, a relatively quick response is characteristic: the result can be obtained in 20 seconds [ 4 ].
Thermal conductivity sensor
The action of this type of sensor, unlike thermistor and catalytic, is not associated with chemical reactions that occur on the surface of the sensor. The basis of their action is the measurement of the thermal conductivity of gases.
One element of this type of sensor is a metal thread made of tungsten, an alloy of tungsten / ruthenium or nickel / iron. The thread is heated to a temperature of about 250 ° C. Its heat is dissipated in the environment, while the thermal conductivity of the gas affects this process. The thermal conductivity of gases varies over a very wide range, and the temperature of the wire will vary in accordance with the nature and concentration of the gas. A change in the temperature of the filament can be detected by a change in its resistance, as well as for other calorimetric sensors [ 2 , 4 ].
For many years, such sensors have been successfully used as gas chromatography detectors and as gas sensors in industry.
Thermal conductivity sensors are used when the expected gas concentration is relatively high. Since their action does not depend on the occurrence of a chemical reaction, they can be used in an inert gas environment, for example, to monitor the content of combustible gases in vessels after they have been filled with nitrogen. They can also be used to determine the inert gases themselves, such as nitrogen, helium, argon and carbon dioxide. Thus, thermal conductivity sensors have their own field of application, which differs from the field of application of catalytic gas sensors, but at the same time supplements it.
3.5. Biosensors
A biosensor is a device that includes a biological sensitive element that is closely connected to or integrated with the transducer [ 21 ]. The biosensor serves to generate a digital electrical signal proportional to the concentration of a particular chemical compound or series of compounds. This connection of two opposing disciplines made it possible to combine the specificity and sensitivity of biological systems with the computing power of a computer. The rapidly growing biosensor technology in recent years already offers new effective tools that predict a radical change in our approach to classical chemical analysis.
The modern biosensor concept is largely associated with the ideas of Leland Clark Jr. and co-authors developed in 1962 . The authors suggested that if enzymes could be immobilized on electrochemical sensors, then such “enzyme electrodes” would expand the range of analytical capabilities of the base sensor. The subsequent active work gradually expanded the horizons of this area. Her current state is characterized to some extent by the following potential sensors and transducers, which can be used in the construction of biosensors [ 2 , 4 , 21 ]:

In real sensors, so far, not all possible combinations of these elements are used. The development of biosensors is due to the efforts of researchers in several directions. The configurations of biosensors described so far are based on a fundamentally new combination of previously well-known and unrelated approaches [ 21 ]. In the future, in order to meet specific requirements, apparently, more attention will be paid to the engineering study of both the entire device and its components. This may require new biochemical reactions and the improvement of known reactions, for example, using genetic engineering and chemical methods. Biosensors will be designed together with a suitable detector, and not tied to random results from previous work.
So, the term biosensor means a device in which the sensitive layer contains biological material: enzymes, tissues, bacteria, yeast, antigens / antibodies, liposomes, organelles, receptors, DNA. This layer responds directly to the presence of the detected component and generates a signal depending on the concentration of this component.
Structurally, the biosensor is similar to other types of chemical sensors and consists of two transducers (biochemical and physical) that are in close contact with each other. In this case, the biochemical converter, or biotransducer, performs the function of a biological recognition element, converting the component to be determined, or rather, information about chemical bonds into a physical or chemical property or signal, and the physical converter allows you to register this signal. The presence of a biomaterial with unique properties in the device allows high selectivity to determine the desired compounds in a complex mixture, without resorting to additional operations associated with the use of other reagents.
As transducers, any of those mentioned in this article can be used: electrochemical, spectroscopic, thermal, piezoelectric, on surface acoustic waves, and integrated optical.
The action of biosensors is based on the most important chemical reactions of living organisms: antibody / antigen, enzyme / substrate, receptor / hormone reactions. Such reactions are used to obtain highly selective and sensitive biosensors for specific analytes. To illustrate highly selective reactions occurring between biological molecules, a mechanism called the “key-lock” is proposed.
To explain the principle of action of biosensors, the circuit shown in Fig. 4. This scheme is quite universal and applicable to any types of sensors in which the reagent has an affinity for an individual substance. To illustrate highly selective reactions occurring between biological molecules, a mechanism called the “key lock” is proposed.
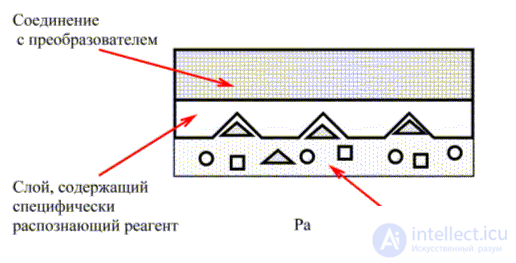
Fig. 4. The biosensor.
In biosensors, the recognition reagent is usually a macromolecule immobilized inside the membrane or chemically bonded to a surface that is in contact with a solution of the analyte. A specific chemical reaction takes place between the reagent and the analyte. This can be either direct interaction of the reagent with the analyte, as in the case of the antigen / antibody reaction, or a catalytic interaction of the immobilized enzyme with the analyte to form an easily detectable product.
Of great interest, for example, are biosensors based on microorganisms immobilized on the membrane, which serve as an element of the so-called microbial sensor [ 21 ] . In particular, an amperometric ammonia sensor based on immobilized nitrifying bacteria and Clark’s oxygen electrode is used in environmental issues.
It should be noted that in recent years lichenoindication monitoring of the air environment has begun to actively develop and apply [ 15 ]. Lichenoindication methods are based on the individual reaction of various species of lichens to the action of atmospheric pollutants. The long-term response of these bioindicator organisms even to microdoses of pollutants, manifested in morphological changes, a change in species composition, and low intrinsic variability, determine their widespread use as bioindicators of air conditions. The results of lichenoindication studies give an integrated assessment of the degree of air pollution over a long period of time and can serve as a good addition to the sanitary-hygienic assessment of environmental conditions. Lichens are very sensitive to chemical pollution and can be good indicators of the state of the environment, both on their own and as a sensitive element of biosensors.
If we take into account the whole variety of enzymes present and acting in living organisms and which are potential biological converters, then the number of biosensor structures existing today can be increased by tens or even hundreds of times. The main difficulties are related to the calibration of biosensors and the reliability of their readings. To improve the latter indicator, in particular, a multisensor system consisting of a number of biochips can be used.
In general, the metrological characteristics of biosensors are quite acceptable. The relative standard deviation of the determined concentration is not worse than 10-12%, while the lower boundary of the determined contents reaches 10 -10 -10 -15 mol / L. Some biosensors operate on a “no-yes” principle, which is acceptable when determining the presence of ultra-small amounts of highly toxic substances in environmental objects. If the determined components are in a complex mixture or matrix, or are similar in their properties, then chromatographic separation methods are used in the analysis.
Note that biosensors are widely used not only in chemistry, but also in biotechnology, medicine and ecology. Promisingly, their application in the electronic industry and security systems, for example, in transport (primarily in air transport), in the coal industry, etc. Numerous accidents, catastrophes, and terrorist attacks of recent years urgently require the accelerated implementation of promising scientific developments in critical areas of life.
3.6. Optical chemical sensors
Optical chemical sensors are one of the most important categories of chemical sensors. Depending on the type of optical sensors, their action is based on the following principles [ 3 - 6 , 9 - 12 , 14 , 16 , 17 ]:
§ light absorption (absorption);
§ reflection of the primary (incident) light flux;
§ luminescence.
In this case, the dependences of the optical properties of the media (refractive index, reflection coefficient, etc.) on the concentrations of the substances to be determined are used.
Consider the fundamental phenomena underlying the action of optical chemical sensors.
Absorption The ability of a substance to absorb optical radiation depends on the structure of atoms (molecules), as well as on the state of aggregation of the substance, its concentration, layer thickness, wavelength and other factors.
The basic laws of absorption of optical radiation, on which the application of the absorption effect for research and analysis of matter is based, are the Bouguer-Lambert law and Beer's law [ 11 , 12 , 14 , 16 ].
According to the first law, if the medium is homogeneous and its layer of thickness l is perpendicular to the monochromatic light flux with intensity I 0 , then the intensity I of the transmitted light is determined by the formula:
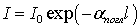 . (2)
. (2)
In the formula (2) 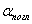 Is the absorption coefficient, which for a given substance depends on the wavelength λ of the incident monochromatic radiation. In cases where the scattering of light cannot be neglected, it is necessary to take into account its contribution to the total attenuation
Is the absorption coefficient, which for a given substance depends on the wavelength λ of the incident monochromatic radiation. In cases where the scattering of light cannot be neglected, it is necessary to take into account its contribution to the total attenuation 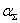 intensities of light transmitted through the medium:
intensities of light transmitted through the medium: 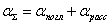 .
.
According to Beer's law, each molecule (or atom) absorbs the same part of the incident radiation, therefore, the absorption is proportional to the number of particles of the absorbing substance N :
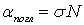 , (3)
, (3)
where N is the concentration of the analyte; σ is the absorption cross section of the analyte at a given incident radiation wavelength  .
.
If both laws are satisfied, then the combined Bouguer-Lambert-Baire law is valid:
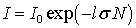 . (4)
. (4)
In case of concentration change 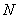 of the studied substance along the path of propagation of light radiation, the Bouger-Lambert-Baer law in integral form is used in the calculations [ 18 ]:
of the studied substance along the path of propagation of light radiation, the Bouger-Lambert-Baer law in integral form is used in the calculations [ 18 ]:
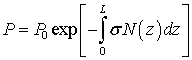 , (5)
, (5)
Where P and P 0 - the power of light radiation at the output of the sensor cell in the presence and absence of the test substance, respectively; L is the thickness of the layer of the investigated medium (corresponding, for example, to the length of the sensor cell); 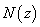 - the concentration distribution of the analyte along the z axis (along which the laser radiation propagates).
- the concentration distribution of the analyte along the z axis (along which the laser radiation propagates).
Reflection . When the light flux falls to the interface between two media, part of its radiation is reflected back. The nature of the reflection depends on the properties of the media and the size of the irregularities at the interface between these media. The intensity of the reflected light is determined by the electronic structure of atoms, molecules and ions in the surface layer of the substance, the processes of absorption and multiple scattering in it, and also depends on the wavelength of the incident light, because 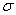 in (2) - (5) can depend on
in (2) - (5) can depend on 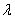 . This allows you to use the reflection effect to study the composition and structure of the surface layers of a solid and turbid media, as well as to identify adsorbed compounds.
. This allows you to use the reflection effect to study the composition and structure of the surface layers of a solid and turbid media, as well as to identify adsorbed compounds.
To study thin films, the method of impaired total internal reflection is used, based on the reflection, for example, of IR radiation at the interface of two media in optical contact (at a distance of the order of molecular forces). In this case, the substance absorbs light of characteristic wavelengths and reflects in the rest of the spectrum.
Luminescence . This phenomenon is a luminescence of a substance that occurs after it absorbs excitation energy, and is excess radiation compared with the thermal radiation of a body at a given temperature.
Photoluminescence , the source of which is light, is of the greatest importance for determining the composition of the medium. Photoluminescence is characterized by absorption and luminescence spectra, polarization, energy yield (ratio of energy emitted by a substance in the form of luminescence to absorbed energy), quantum yield (ratio of the number of emitted quanta to the number of absorbed) and kinetics.
The most widely used analysis is based on photoluminescence excited by UV radiation, the source of which is mercury-quartz and xenon lamps, as well as lasers. Registration of luminescence is carried out visually and photoelectrically (using a spectrophotometer). The characteristics of photoluminescence allow us to draw conclusions about the presence of certain substances in the studied samples and their concentration. Quantitative analysis is based on the dependence of the luminescence intensity on the amount of luminescent substance.
Most often, optical chemical sensors are classified according to the type of principles of their action: absorption sensor, reflection sensor, luminescence sensor, combined sensor, etc.
The structure of optical chemical sensors . In optical chemical sensors operating on physical principles, the analytical signal is caused not by the chemical interaction of the detected component with the sensitive layer, which acts as a transducer, but by a measured physical parameter: the absorption, reflection, or luminescence of light, etc.
The fiber optic sensor is usually made of quartz glass, plastic or glass and is surrounded by an optical insulator - a shell having a lower refractive index than the core. Plastic and glass fibers are much cheaper than silica glass fibers, but the scope of silica fibers is much wider: they can be used in the ultraviolet region of the spectrum, where other materials absorb radiation.
Both single optical fibers and bundles of many optical fibers are used. Optical fibers allow the transmission of optical signals over very large distances and, therefore, are ideal for those days when the object of analysis is remote from the researcher. In addition, they can be bent (however, the bending angle should not be too sharp), and therefore they can be used in a wide variety of optical photosensitive devices, such as flow cells for continuous monitoring.
Integrated Optical Sensor . In our opinion, integrated optical chemical sensors are the most promising among optical chemical sensors [ 9 - 12 , 17 , 18 , 19 ]. The principle of operation of absorption-type integrated optical chemical sensors is based on recording changes in the intensity of laser radiation of the waveguide mode propagating through the gaseous or liquid medium under investigation (located next to the sensor) at the wavelengths characteristic of this substance.
In fig. 5 is a schematic cross-sectional view of a simple three-layer integrated optical thin-film waveguide chemical sensor. It is formed by three media: air 1, film 2 and substrate 3 with refractive indices of the media 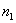 ,
, 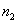 and
and 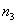 respectively. To provide guiding properties, refractive indices
respectively. To provide guiding properties, refractive indices 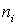 waveguide media are selected from the condition:
waveguide media are selected from the condition: 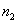 >
> 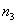 >
> 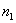 .
.
In the optical-beam approximation, laser radiation introduced into a regular waveguide propagates along the waveguide in the form of plane waves moving along a zigzag path and experiencing total internal reflection at the waveguide boundaries [ 9 - 12 , 14 , 16 - 19 ]. The optical energy of the mode does not weaken as a result of interference of the waves reflected at the boundaries of the waveguide if the total phase change in the vertical direction is a multiple of 2 π . In this case, they say that the resonance condition is satisfied. The field strength of the waveguide mode in waveguide layer 2 has a sinusoidal distribution, and in media 1 and 3 it is exponential. Typically, localized TE modes are used, the field of which decays exponentially in air and the substrate with distance from the waveguide layer 2.
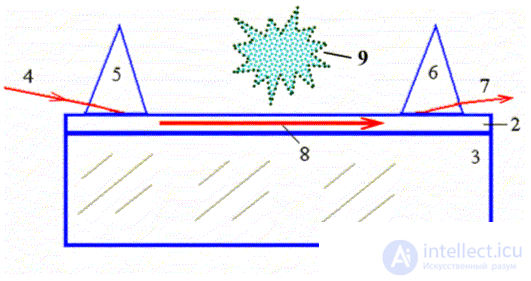
Fig. 5. Integrated optical waveguide chemical sensor.
The optical waveguide sensor cell is formed by media 1–3: 1 — cover layer (air), 2 — waveguide layer (film); 3 - substrate; 4, 7 - input and output laser radiation; 5, 6 - prisms of input and output of laser radiation; 8 - guided waveguide mode; 9 - the investigated environment.
If gas 9 (or another medium under study, for example, vapor, liquid) appears near the waveguide in air (at the interface between media 1-2), which has a characteristic absorption line that matches the wavelength of the laser radiation, then power attenuation will be observed waveguide mode. It is this effect that underlies the operation of the integrated optical chemical sensor of the absorption type.
The waveguide layer 2 can be made of polystyrene, gelatin and a number of other optically transparent materials. For example, a layer of Ta 2 O 5 is applied to the substrate by cathodic sputtering. The integrated optical sensor can be created on the basis of a diffuse waveguide made by doping PbO 2 into a glass substrate. The thickness of the film (waveguide layer 2), as a rule, is comparable with the wavelength of monochromatic light 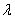 and in the visible range usually does not exceed 1-5 micrometers.
and in the visible range usually does not exceed 1-5 micrometers.
The substrate 3 of the waveguide was usually a plate several millimeters thick, for example, made of glass with a high surface finish (the rms surface roughness is less than 100 Å). The length of the sensor cell of the integrated optical chemical sensor is determined by the distance between the input and output of radiation through prism communication devices and can vary from a few millimeters to meters [ 9 - 12 ]. For input and output of laser radiation, prisms with a refractive index greater than that of media 1-3 forming a waveguide are used.
In fig. Figure 6 shows a diagram of an intelligent digital measuring system used to test the detection abilities of an integrated optical chemical sensor based on a diffuse waveguide made by doping PbO 2 into a glass substrate [ 12 , 17 , 18 ]. A helium-neon laser 1 with a wavelength was used as a source of coherent radiation 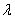 = 632.8 nm, which coincides with one of the absorption bands of ammonia. The laser beam is separated by a translucent mirror 2 into the reference and touch beams. The sensor beam is introduced into the integrated optical waveguide sensor cell 3 through the input prism at an angle that corresponds to the resonant excitation of the TE 0 mode.
= 632.8 nm, which coincides with one of the absorption bands of ammonia. The laser beam is separated by a translucent mirror 2 into the reference and touch beams. The sensor beam is introduced into the integrated optical waveguide sensor cell 3 through the input prism at an angle that corresponds to the resonant excitation of the TE 0 mode.
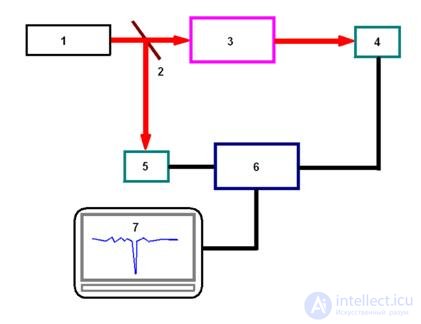
Fig. 6. Diagram of an intelligent digital measuring system with an integrated optical chemical sensor.
The radiation introduced into the waveguide propagates through the waveguide ( Fig. 5 ), partially penetrating into the air, and in the presence of ammonia at the output of the output prism, a decrease in the intensity of the signal detected by the sensor photodetector 4. The signal of the reference beam was detected by the second photodetector 5. Silicon photodetectors were used photodiodes FD-256. These photodiodes are intended for use as an optical radiation receiver in the range of about 0.4 μm to 1.1 μm. The mode of operation of photodiodes, as a rule, is photodiode (with an external bias source). At low signal-to-noise ratios, it is preferable to use photomultiplier tubes. The signals from the photodetectors were fed to an electronic comparison circuit 6. After analog-to-digital conversion, the signal was recorded and processed by computer 7. To record the results of experiments in digital form, for example, a virtual laboratory of the PC - LAB type can be used , the capabilities of which can be expanded by further mathematical processing experimental data [ 18 , 19 ].
To enter and output laser radiation into the waveguide sensor, both prisms and diffraction gratings can be used. You can use the end input of laser radiation. The choice of a specific type of waveguide and methods for input and output of laser radiation into an integrated optical waveguide is determined by the design of the sensor, the type of test substance, and the technological requirements for the sensor.
We emphasize that the features of the operation of various integrated optical sensors in the visible wavelength range are still poorly studied. For example, there is no reliable data on the interaction of ammonia molecules, both with the surface of a specific sensor and with the surface layer of the sensor in the field of laser radiation of the waveguide mode. Although already in the first works on integrated-optical sensors, the possibilities of complex interaction of the detected substance and the sensor were noted (see, for example, [ 8 , 9 ]). So the following phenomena are possible: a change in the dielectric constant of the surface layer at the moment of the action of the detected substance (the effect can be reversible and irreversible), a nonlinear process of interaction of the waveguide mode field with the detected substance, amplification of the detected effect with the help of an additional (chemical-transducer) layer, which contains immobilized molecules of a substance that selectively and reversibly reacts to the presence of a test substance. Such a layer may be the waveguide layer of the integrated optical sensor. Undoubtedly, a comprehensive study of all these phenomena requires an interdisciplinary approach and rather laborious and expensive experiments.
Ammonia gas was used to test the detection capabilities of the integrated optical
продолжение следует...
Часть 1 Portable digital gas sensors. electrochemical analog sensitive elements
Часть 2 - Portable digital gas sensors. electrochemical analog sensitive elements
Comments
To leave a comment
Sensors
Terms: Sensors